Electronic Data Capture Systems Market: Trends, Growth, and Future Prospects Size, Share, 2032

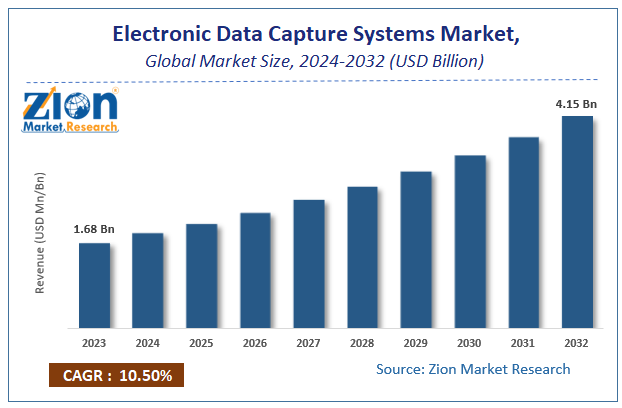
By the end of 2032, the global market for electronic data capture systems is expected to have grown from its 2023 valuation of USD 1.68 billion to USD 4.15 billion. Over the course of the projected period, the market is anticipated to expand at a CAGR of 10.5%. The growth factors, barriers, and effects on demand of the worldwide electronic data capture systems market are examined in this study for the period of forecasting. Additionally, it will assist in navigating and investigating the emerging opportunities in the field of electronic data capture systems.
✈👉Get a Free Sample: 🚀https://www.zionmarketresearch.com/sample/electronic-data-capture-systems-market
Overview of the Global Market for Electronic Data Capture Systems
Web-based software called an electronic data capture system is used to retain all patient data gathered from clinical trials and tests. After being reported on paper, the data is recorded and stored on an electronic data capture system. According to studies, about 80% of clinical trials end in failure because of the growing complexity of data handling, which also delays drug commercialization due to unmet regulatory requirements. Since clinical research centers and businesses need to do more work in less time, these electronic data collection systems are an ideal substitute since they save time, provide accuracy, and produce findings quickly. Consequently, there is a growing need for electronic data capture systems in clinical research institutions and pharmaceutical businesses.
The Electronic Data Capture (EDC) systems market is experiencing significant growth due to the increasing need for efficient, accurate, and streamlined data collection processes in sectors such as healthcare, pharmaceuticals, clinical research, and life sciences. EDC systems automate the process of collecting, storing, and analyzing data, significantly improving accuracy and reducing errors when compared to traditional paper-based methods. With the growing demand for digital solutions in clinical trials, research studies, and patient data management, the EDC systems market is poised for continued expansion.
Growth Factors for the Global Electronic Data Capture System Market
One of the methods that academics and medical professionals favour for clinical investigations is electronic data acquisition. Improvements in information management and the need to precisely and clearly compile technical, medical, and scientific data are driving the need for electronic data capture, which is in turn driving the expansion of the global market for electronic data capture systems. Because e Clinical systems offer a variety of workflow options, there is substantial growth potential for clinical trials, medical devices, and electronic medical records. These benefits include the removal of data inconsistencies and guidance on how to interpret new discoveries in a medical context. Other benefits of using electronic data capture technologies include enhanced technical and scientific data compilation and advanced statistical analysis, which are necessary for regulatory approval of clinical investigations.
These are believed to enhance the entire approval process, which will lead to more future users of EDC methods. Each of these elements contributes equally to the expansion of the global market. Rapid technological advancement is the primary driver of the worldwide electronic data capture market’s growth. Furthermore, several providers are always creating new EDC strategies in order to keep up with market developments. During the forecast period, this is probably going to help the expansion of the global market for electronic data capture systems. However, for start-ups and small businesses, purchasing an electronic data capture system can be a significant financial commitment; as a result, the market may see a little downturn in these situations. Additionally, the expansion of the global market for electronic data capture systems may be hampered by the abundance of substitute choices, such as software-as-a-service, which supports clinical research and functions similarly.
Companies attempted to create their reports using electronic data capture in an effort to expedite the collection of COVID-19 data and facilitate a seamless process throughout the pandemic. Research on COVID-19 may benefit from the characteristics of electronic data gathering systems in order to better understand the virus and stop similar outbreaks in the future. The global market for electronic data capture systems rose faster as a result of the increased use of EDC during the epidemic.

Market Segmentation for Electronic Data Capture Systems Worldwide
The components, delivery mode, development phase, end-user application, and geographic segments make up the global market for electronic data capture systems. The worldwide market for electronic data capture is separated into software and services based on componentry. The market is divided into three segments based on the mode of delivery: cloud-based, licensed enterprise, and web-hosted. Phase 1, Phase 2, Phase 3, and Phase 4 comprise the development phase portion. Hospitals, CROs, academic institutions, pharmaceutical and biotech companies, and medical device makers make up the market’s end-user application segment.
✈👉Directly Purchase a copy of the report with TOC: 🚀https://www.zionmarketresearch.com/toc/electronic-data-capture-systems-market
Market for Electronic Data Capture Systems: Report Purpose

Regional Analysis of the Global Market for Electronic Data Capture Systems
It is anticipated that North America will continue to dominate the global market for electronic data capture systems throughout the forecast period. Such data capturing devices are becoming more and more popular due to the government’s strict rules for the safe and discreet handling of healthcare information. Another significant potential opportunity is the growing awareness of the need to handle clinical data just to satisfy regulatory requirements. Additionally, the market is anticipated to develop profitably due to the high number of pharmaceutical businesses in the North American region, which has led to a better use of electronic data capture systems. The availability of numerous clinical research businesses that supply medical devices and clinical trial solutions to different pharmaceutical corporations is another reason why Asia Pacific is expected to grow significantly. Furthermore, the global market for electronic data capture systems is anticipated to develop significantly in the area as a result of advantageous government policies and technical advancements occurring in Europe.
Key Market Segments
- By Component
- By End-User
- By Deployment
- By Region
Market Drivers
- Increased Demand for Clinical Trials: With the rise in clinical research activities and the increasing number of clinical trials worldwide, EDC systems are essential for managing large volumes of data and ensuring compliance with regulatory standards.
- Regulatory Requirements: Governments and regulatory bodies are enforcing stricter guidelines for data management in clinical trials, including the use of electronic systems to ensure data integrity and security. This has spurred the demand for EDC systems.
- Technological Advancements: The advancement of cloud computing, data analytics, and automation is driving the growth of cloud-based EDC systems, making them more accessible and affordable for smaller organizations and healthcare providers.
- Cost and Time Efficiency: EDC systems streamline data entry, collection, and analysis processes, reducing the time and cost associated with manual data handling. This is a key driver for adoption in clinical trials and research studies.
Challenges
- Data Security and Privacy Concerns: Ensuring the privacy and security of sensitive patient and clinical trial data remains a significant challenge. Healthcare providers and research organizations must adhere to strict regulations like HIPAA and GDPR to protect patient data.
- Integration with Existing Systems: Integrating EDC systems with existing software and infrastructure can be complex, especially for organizations with legacy systems. This can lead to higher costs and longer implementation times.
- Training and Adoption: Successful adoption of EDC systems requires proper training and a cultural shift in organizations that are accustomed to manual processes. Resistance to change and a lack of skilled personnel can hinder the market’s growth.
Key Players
The EDC systems market is highly competitive, with several prominent players offering a wide range of solutions. Key players in the market include:
- Medidata Solutions, Inc.
- Veeva Systems Inc.
- Parexel International Corporation
- Oracle Corporation
- IBM Corporation
- Bio-Optronics
- Cognizant Technology Solutions
- CRF Health
- Syneos Health
- Bioclinica
Market Size and Forecast
The global electronic data capture systems market was valued at approximately $5.2 billion in 2023 and is projected to grow at a CAGR of 13.1%, reaching $12.3 billion by 2030. Growth drivers include increased clinical research activities, the rise of cloud-based solutions, and technological advancements in data analytics.
Future Trends
- Integration with Artificial Intelligence (AI) and Machine Learning (ML): The integration of AI and ML algorithms into EDC systems will help automate data analysis, detect patterns, and improve decision-making during clinical trials and research studies.
- Mobile and Remote Data Collection: With the growing trend of remote patient monitoring and decentralized clinical trials, EDC systems will increasingly offer mobile-friendly platforms for collecting data in real-time from patients and research participants.
- Advanced Data Analytics: EDC systems will continue to evolve by incorporating advanced data analytics tools to help researchers and healthcare providers derive insights from large datasets, improving decision-making and operational efficiency.
- Focus on Data Interoperability: To streamline data management, future EDC systems will increasingly prioritize interoperability with other healthcare systems, such as electronic health records (EHRs) and laboratory information systems (LIS), to ensure seamless data flow across platforms.
Conclusion
The electronic data capture systems market is poised for significant growth as demand for accurate, efficient, and secure data collection methods continues to rise in clinical trials, healthcare, and research. Technological advancements, regulatory pressure, and the push toward digital transformation in healthcare are the primary drivers of this market’s expansion. Companies that innovate in data security, AI integration, and cloud-based solutions are likely to lead in the competitive landscape. Despite challenges related to data security and system integration, the future of the EDC market remains strong, offering substantial opportunities for both established players and new entrants.
✈👉Enquiry for buying: 🚀https://www.zionmarketresearch.com/inquiry/electronic-data-capture-systems-market
✍Browse other trend reports:
Automotive Ball and Roller Bearings Market
Automotive Metal Die Casting Market
https://www.linkedin.com/pulse/thermal-printing-market-size-share-trends-analysis-yhclc
https://www.linkedin.com/pulse/thermal-scanners-market-size-share-growth-report-8cfvc
https://www.linkedin.com/pulse/portable-pressure-washer-market-size-share-growth-lv4bc
https://www.linkedin.com/pulse/wire-cable-materials-market-size-share-trends-analysis-tcbic
https://www.linkedin.com/pulse/electronic-data-capture-systems-market-growth-size-kbqmc
📞Contact Us:
Zion Market Research212
USA/Canada Toll Free: 1 (855) 465–4651
Network: 1 (302) 444–016611\
📲Web: https://www.zionmarketresearch.com/
👉Blog: https://zmrblog.com/
Comments
Post a Comment